Influence of different concentrations of jasmonic acid on in vitro development of Catasetum fimbriatum Lindl. (Orchidaceae)
Abstract
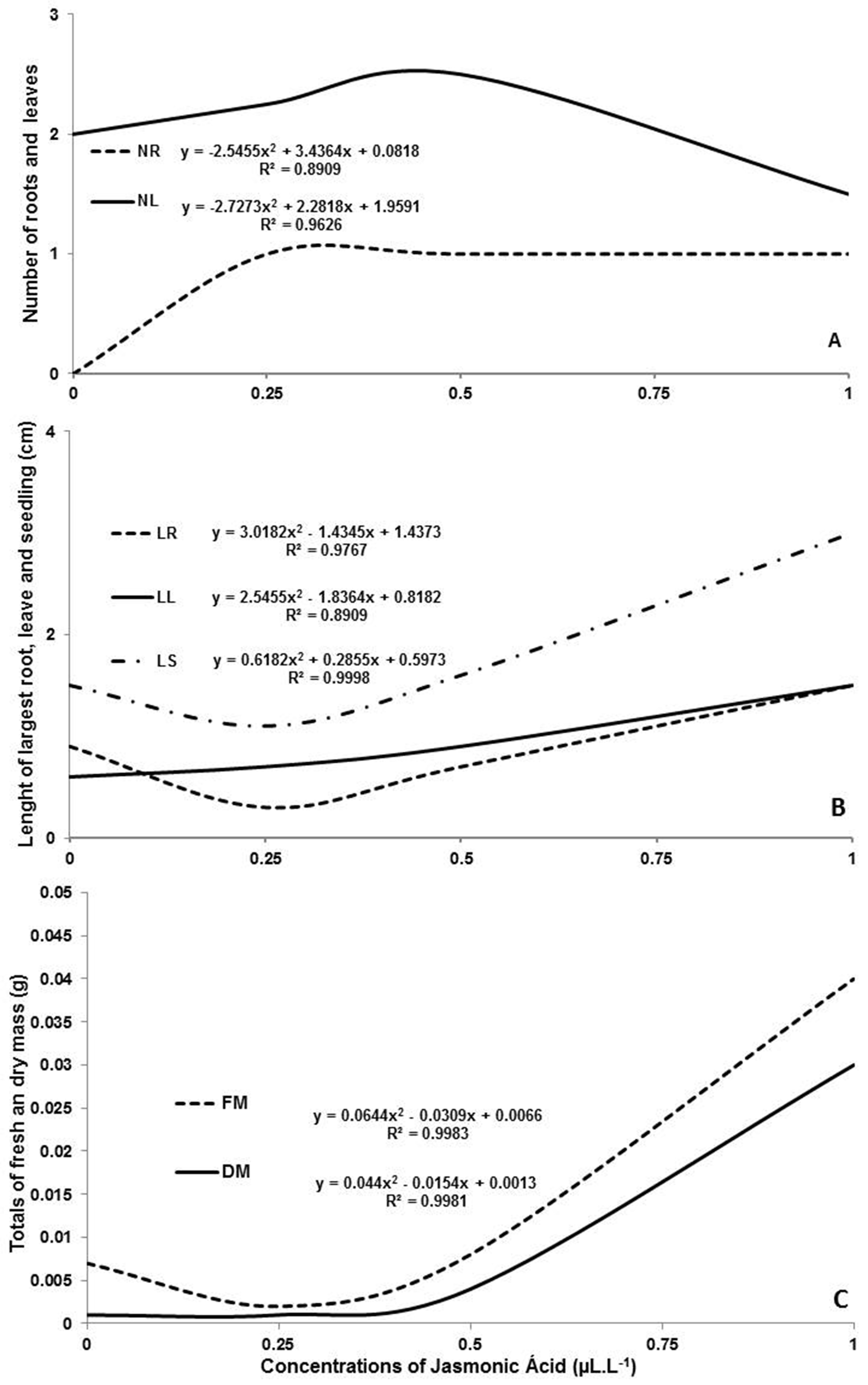
In vitro seeding constitutes an indispensable tool for propagation of the main commercial species of orchids. This study aimed to analyze the in vitro development of Catasetum fimbriatum under different concentrations of jasmonic acid in Murashige & Skoog culture medium compound of ½ macronutrients. After 180 days of cultivation, concentration of 0.25 and 0.50 μL • L-1 caused a significant increase in number of roots and leaves. The concentration of 1.00 μL • L-1 showed the best result for the length of longest root and largest leaf, the total length of seedling, and the total fresh and dry masses. Nevertheless, the use of this plant regulator on in vitro culture media still requires further investigations to determine the optimal concentration in order to obtain desirable phytotechnical characteristics in different orchid species.
References
Ayres M., Ayres J.R.M., Ayres D.L., Santos A.S. 2007. BioEstat 5.3 – Aplicações Estatísticas nas Áreas das Ciências Biológicas e Médicas: Sociedade Civil Mamirauá, Belém.CNPq, Brasília.
Bicalho H.D., Barros F. 1998. On taxonomy of Catasetum subsection Isoceras: Lindleyana 3: 87–92.
Borin L., Cavalcante V.R., Paioli R.R., Pedroso-de-Moraes C. 2015. Different concentrations of jasmonic acid on the in vitro growth of Cattlianthe “Jewel Box” (Orchidaceae). J. Biol. Nature 3: 145–151.
Cardoso J.C. 2014. Levantamento de espécies da família Orchidaceae em Botucatu: potencial ornamental e cultivo. Horticultura Brasileira 32: 7–13.
Dezan L.F., Canassa F., Souza-Leal T., Diogo J.A., Massaro R., Cordeiro G.M., Pedroso-de-Moraes C. 2012. Crescimento in vitro de Schomburgkia gloriosa Lindl. em meio de cultivo simplificados. Idesia (Arica) 30: 53–58.
Dodson C.H. 1975. Dressleria and Clowesia: a new genus and an old one revived in the Catasetinae. Selbyana 1: 130–137.
Facchini P.J., Johnson A.G., Poupart J., Deluca V. 1996. Uncoupled defense gene expression and antimicrobial alkaloid accumulation in elicited opium poppy cell cultures. Plant Physiol. 111: 687–697.
Fernandes M.R., Barboza M.P., Souza-Leal T., Pedroso-de-Moraes C. 2012. Morfobiometria carpo seminal e germinação de Lafoensia pacari A. St. Hil. (Lythraceae) exposta a diferentes concentrações de GA3. Semina: Ciências Agrárias 3 (suppl. 1): 2571–2584.
Hoehne F.C. 1938. As plantas ornamentais da flora brasílica. Boletim de Agricultura, São Paulo 1: 247–273.
Hoehne F.C. 1949. Iconografia de Orchidaceae do Brasil. Instituto de Botânica, São Paulo.
Hsu C.C. 2003. Protocorm-like body induction and plant regeneration from etiolated leaves of in vitro Phalaenopsis. PhD thesis. Institute of Tropical Agriculture and International Cooperation, University of Science & Technology, Pingtung.
Joly A.B. 1998. Botânica: introdução à taxonomia vegetal. 12th ed. Companhia Editora Nacional, São Paulo.
Kerbauy G. 2008. Fisiologia vegetal. 2nd ed. Guanabara Koogan, Rio de Janeiro.
Koda Y. 1992. The role of jasmonic acid and related compounds in the regulation of plant development. Int. Rev. Cytol. 135: 155–159.
Linares A.M.P., Hernandes C., França S.C., Lourenço M.V. 2010. Atividade fitorreguladora de jasmonatos produzidos por Botryosphaeria rhodina. Horticultura Brasileira 28: 430–434.
Maksymiec W., Krupa Z. 2007. Effects of methyl jasmonate and excess copper on root and leaf growth. Biol. Plant. 51 (2): 322–326.
Moraes C.P., Almeida M. 2004. Influência climática sobre a plasticidade fenotípica floral de Catasetum fimbriatum Lindley. Ciência & Agrotecnologia 28: 942–948.
Murashige T., Skoog F.A. 1962. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Parthier B. 1990. Jasmonates: hormonal regulators or stress factors in leaf senescence? J. Plant Growth Regul. 9: 1–7.
Pedroso-de-Moraes C. 2000. Cultivo de orquídeas. Biblioteca Duse Rüegger Ometto, Araras, SP.
Pedroso-de-Moraes C. 2002. Fenologia e anatomia dos órgãos reprodutivos de Catasetum fimbriatum Lindley cultivados sob diferentes intensidades luminosas. 80f. Dissertação (Mestre em Ciências: Fisiologia e Bioquímica de Plantas). Escola Superior de Agricultura Luiz de Queiroz ESALQ-USP, Piracicaba, SP, Brazil.
Pedroso-de-Moraes C., Moura E.R.R., Silva M.C., Abdalla M.A. 2007. As orquídeas e o mercado. Boletim da Coordenadoria das Associações Orquidófilas do Brasil (CAOB) 66: 36–42.
Pedroso-de-Moraes C., Santos N.S., Massaro R., Cordeiro G.M., Souza-Leal T. 2009. Desenvolvimento in vitro de Cattleya tigrina A. Richard. (Orchidaceae) utilizando fertilizantes comerciais. Ensaios e Ciência 13: 57–65.
Pedroso-de-Moraes C., Souza-Leal T., Brescansin R.L., Petini-Benelli A., Sajo M.G. 2012. Radicular anatomy of twelve representatives of the Catasetinae subtribe (Orchidaceae: Cymbidieae). Anais da Academia Brasileira de Ciências 84: 455–468.
Pridgeon A.M., Cribb P.J., Chase M.A., Rasmussen F.N. (eds) 2009. Genera Orchidacearum. Vol. 5. Epidendroideae (Part II). Oxford University Press, Oxford.
Reinboth S., Mollenhauer B., Reinbothe C. 1994. JIPs and RIPs: The regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell 6: 1197–1209.
Romero G.A. 1990. Phylogenetic relationships in subtribe Catasetinae (Orchidaceae, Cymbidieae). Lindleyana 5: 160–181.
Stancato G.C., Bemelmans P.F., Vegro C.L.R. 2001. Produção de mudas de orquídeas a partir de sementes in vitro e sua viabilidade econômica: estudo de caso. Revista Brasileira de Horticultura Ornamental 7: 25–33.
Staswick P.E. 1992. Jasmonate, genes and fragrant signals. Plant Physiol. 99: 804–807.
Ventura G.M., Dias J.M.M., Teixeira L.S., Carvalho S.V., Motoike Y.S., Novais F.R., Cecon R.P. 2002. Organogênese in vitro a partir de gemas apicais e auxiliares de plantas adultas de orquídeas do grupo Cattleya. Revista Ceres 47 (286): 613–628.
Vick B.A., Zimmerman D.C. 1986. Characterization of 12-oxophytodienoic acid reductase in corn. The jasmonic acid pathway. Plant Physiol. 80: 202–205.
Wilen R.W., van Rooijen C.J.H., Pearce D.W., Pharis R.P., Holbrook L.A., Moloney M.M. 1991. Effects of jasmonic acid on embryospecific proceses in Brassica and Linum oil seeds. Plant Physiol. 95: 399–405.
Zimmerman G.K. 1991. Ecological correlates of labile expression in the orchid Catasetum viridiflavum. Ecology 72: 597–608.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
The journal is licensed by Creative Commons under BY-NC-ND license. You are welcome and free to share (copy and redistribute the material in any medium or format) all the published materials. You may not use the material for commercial purposes. You must give appropriate credit to all published materials.
The journal allow the author(s) to hold the copyrights and to retain publishing rights without any restrictions. This is also indicated at the bottom of each article.





